Registration of drugs in Ukraine: documents, procedure, possible difficulties

1. Drugs that are subject to registration
It is required to register all drugs that are produced within and/or imported onto the territory of Ukraine.
There is no need to register only drugs that are produced:
- in pharmacies based on physicians’ prescriptions;
- for medical establishments based on their orders.
2. Documents that are required for registration of drugs
List of documents that are required for registration of drugs depends on the type of drug and whether or not an applicant wants to register active pharmaceutical ingredient (API) or active ingredient that is contained in it along with registration of the drug. Depending on the abovementioned option you choose there can be three types of registration applications and therefore three lists of documents that are to be attached (the specific list of documents also depends on the type of drug and terms of its registration: in many cases it is not required to provide some of the documents listed below).
| Application form #1 (DOWNLOAD) |
Application form #2 (DOWNLOAD) |
Application form #3 (DOWNLOAD) |
| Is used for registration of drugs, including immunological drugs, in accordance with the complete procedure |
Is used for registration of traditional drugs and drugs that are produced in accordance with approved prescriptions |
Is used for registration of API or active ingredient of a drug |
List of documents that are to be attached:
|
List of documents that are to be attached:
|
List of documents that are to be attached:
|
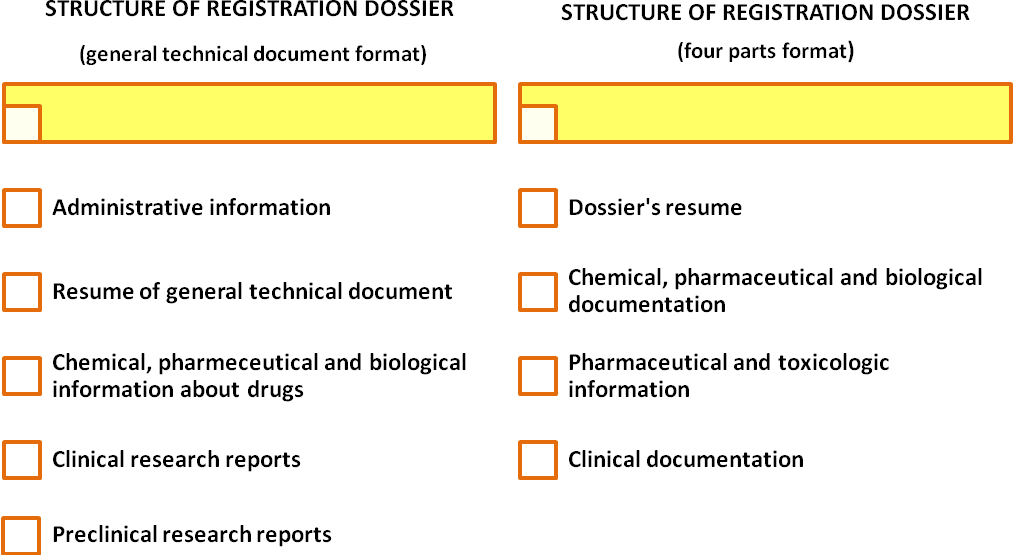
In order for an expertise to be conducted based on application, letter of expertise conduction and recommendations of the Ministry of Healthcare of Ukraine an applicant is required to provide registration dossier of his drug. Depending on the type of the drug and recommendations of the Ministry of Healthcare of Ukraine the registration dossier may have one of the following formats:
3. Procedure of registration
3.1. Submission of application and its consideration
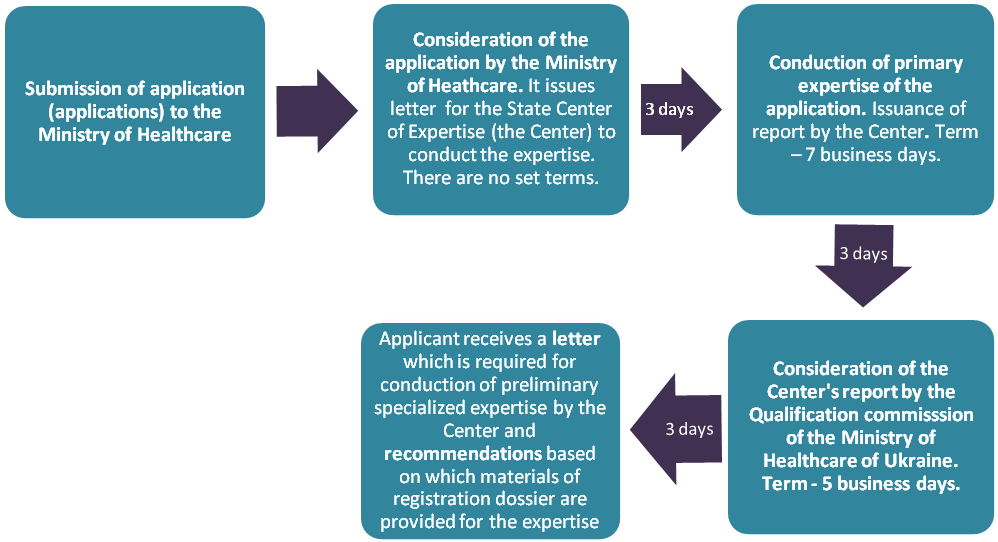
Possible difficulties:
- delays in consideration of documents and their transfer from the Ministry of Healthcare to the Center, from the Center to the Ministry, from the Ministry to an applicant;
- drug registration denial based on the fact of its inclusion into the list of drugs that are prohibited to use;
- detection of inconsistency of the application with the set requirements;
- Ministry of Healthcare reaches the conclusion about necessity to provide application of a different type.
3.2. Preparations for and conduction of drug expertise
Possible difficulties: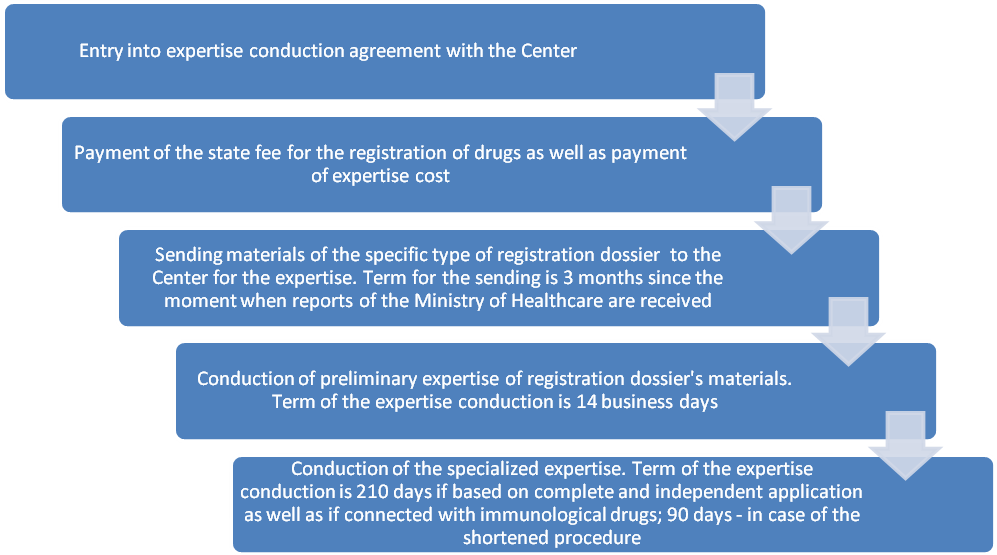
- extension of terms of preliminary expertise conduction when it is required to provide additional data and/or information (term for provision of additional materials equals to 90 business days);
- appointment of additional tests and/or additional expertise (they are paid for additionally);
|
! |
Time that is required for preparation and provision of additional data and/or information as well as for conduction of additional tests (expertise) is not included into the term of preliminary expertise. |
- drug registration denial. Reasons:
а) failure to provide materials of registration dossier for expertise in time;
b) inconsistency of registration dossier’s materials with the set requirements;
c) non-provision of additional materials and/or information within the set term;
|
! |
In case of drug registration denial the registration fee and cost of the expertise are not subject to reimbursement. |
- receiving negative results of specialized expertise and as the result registration denial. Grounds:
a) the drug is harmful and/or it has not therapeutic effectiveness;
b) ingredients of the drug are not in conformity with the information provided in the application;
c) materials of registration dossier are not in compliance with the set requirements;
d) it is found out about unlawful use or referring to information about effectiveness and safety of referent/original drug which contains the same active ingredient within the first 5 years since the date of registration of the drug;
e) the registration will result in infringement of copyrights.
3.3. Issuance of registration certificate

Possible difficulties:
- delays in issuance of registration documents;
- sending of documents’ projects for registration without consideration (if applicant does not provide his comments within 15 days).
4. Key regulatory acts
- Law of Ukraine “On medicinal drugs” (Art. 9)
- Resolution of the Cabinet of Ministers of Ukraine number 376 “On approval of Procedure of state registration (re-registration) of drugs and amount of fee for their state registration (re-registration)” dated 26.05.2005
- Order of the Ministry of Healthcare of Ukraine number 426 “On approval of Procedure of conduction of expertise of drugs registration materials that are provided for the state registration (re-registration) as well as expertise of materials provided for registration of changes made to registration materials during the term of validity of registration certificate” dated 26.08.2005